Quality Pharmaceutical Equipment and Unmatched Service
Why Work With IPP?
IPP Deliver some of the best pharmaceutical equipment and tools on the market. Paired with over 30 experience years collaborating with the pharmaceutical industry. Not only that, but with IPP you’ll have access to Brennan & Co’s comprehensive Service division, so your equipment will always be supported.
Applications / Success Stories
Validated EMS Systems
In addition to providing the Yokogawa paperless system, IPP can also offer a complete turnkey EMS system to the customer, providing the knowledge of the instrumentation required and the experience of the regulations governing the monitoring of Environmental data. The complete package that Irish Power and Process (IPP) offer includes:
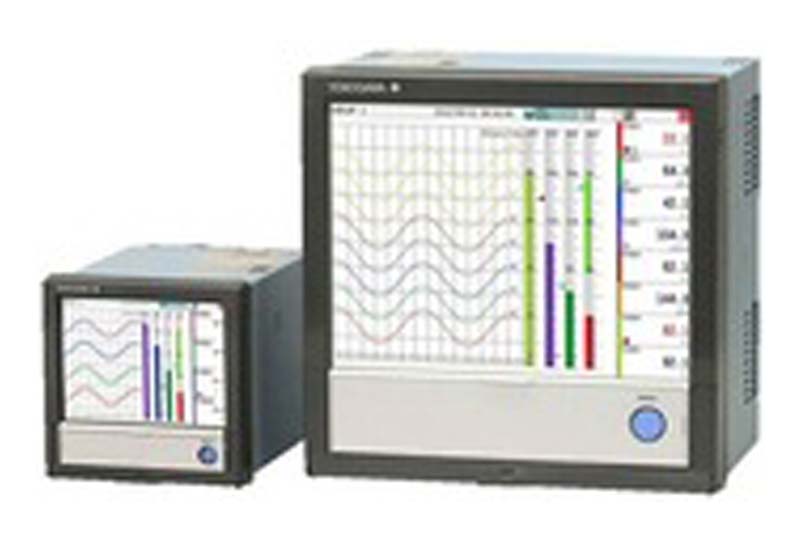
IPP have successfully installed the Yokogawa DXAdvanced R4 Paperless Recorder Data Acquisition system in large numbers throughout Ireland in heavily regulated Pharmaceutical and Medical Device companies, audited by both the FDA and Irish Medicines Board (IMB).

The Solution For measuring critical Process Conditions

When critical process conditions need to be measured securely the Yokogawa GX Series offers the perfect solution. This Data Acquisition system has been successfully installed by IPP in large numbers throughout Ireland in heavily regulated Pharmaceutical and Medical Device companies, audited by both the FDA and Irish Medicines Board (IMB). These validated systems are typically installed in GMP critical processes where accuracy of measurement and security of data is paramount. Such applications include validated EMS licensed Environmental monitoring, sterilisation, lyophilisation and WFI processes.


CONDUCTIVITY CALIBRATION WITH AN INSACAL MASTER METER
Why Calibrate Conductivity?
To be able to get a well documented and traceable means of calibrating your conductivity equipment to the standards required by USP (United States Pharmacopeia) or EP (European Pharmacopoeia). With an INSACAL™ master meter you get a very flexible calibration tool. You can calibrate by closed loop procedures or in an open beaker. This allows you to choose whether your cell is calibrated on-site in-situ or not. If customers use a ”Master Meter” these need to be calibrated.
Measuring Conductivity Correctly In Clean-In-Place (CIP) System
Yokogawa’s FLXA 21 Inductive Conductivity Transmitter has earned a reputation in the recovery of cleaning solutions because it can take measurements with good boundary surface precision over a wide range.
This reduces operating costs and optimises cleaning and sterilising.
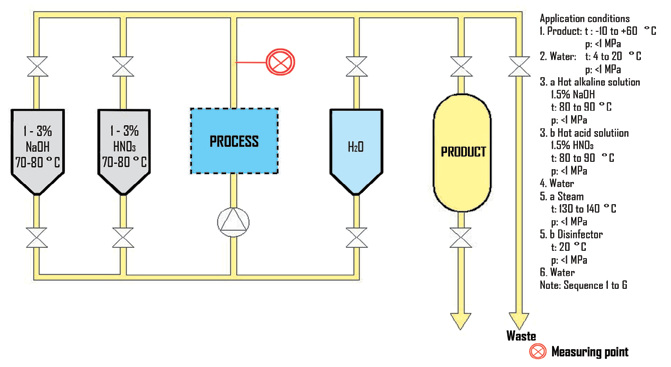
Process Overview
CIP systems depend on the process differentiations of Pharmaceutical, Chemical and Food & Beverage. In addition, the cleaning solutions and chemicals are different. The chemicals that are generally used are hypochlorous acid, caustic soda, and nitrous acid. In a cleaning solution recovery system, the process lines and piping are cleaned with an initial cleaning solution. After that, the cleaning solution is cleaned with fresh water. The cleaning solution is recovered at that point, but since it gradually becomes diluted with fresh water, recovery is discontinued and the solution is discharged through a drainage line when the concentration of the cleaning solution drops below the standard value. The cleaning with different solutions or fresh water, the recovery of the cleaning solution, and the discharge of waste solution are repeatedly carried out. In addition, sterilization with steam or cleaning with distilled water or de-ionized water is performed as a final step in some cases.
Set Up SC450 To Read Temperature
There are two choices for programming the ISC450 for Percent Concentration measurement. The simplest, is to select one of the “built-in” tables already provided in the ISC40. However, if none of these tables meet the application requirements, then the second option of creating your own table specific to your needs can be used.

ISC450 – PROGRAMMING PERCENT CONCENTRATION PREPROGRAMMED CURVES
pH Control And Wash System For 2 – Wire Transmitter Using Controller With PLC Function
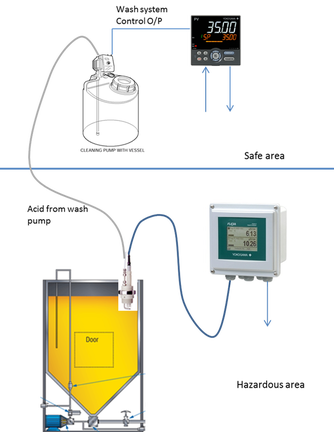
By using the Yokogawa UT35A, it provides a cost effective solution for pH applications.
Application Outline
A perennial issue encountered in process pH measurements is the coating of the electrode surface by a component of the process fluid. In order to function correctly a pH electrode needs to make a good contact to the liquid it is measuring. Any deposit forming on the electrode forms a barrier between the electrode sensing surface and the fluid being measured, depending on the type and degree of deposit it will at least slow the response and, in time, prevent the electrode functioning at all. The ramifications of this for users are significant: